European Commission Closes Antitrust Investigation Following The Withdrawal By Edwards Lifesciences Of Its Disputed Company Policy.
Brussels; February 2026: The European Commission today (16th February 2026) has decided to close its antitrust investigation into allegedly anticompetitive behaviour by Edwards Lifesciences, a producer of medical devices for cardiovascular applications. The closure of the investigation follows the company’s withdrawal of its so-called Global Unilateral Pro-Innovation (Anti-Copycatting) Policy (UPIP). Hence, the UPIP is no longer applicable and has been removed from the company’s website.
In September 2023, the Commission carried out unannounced inspections at Edwards Lifesciences’ premises in the EU. The purpose of the inspections was to investigate whether conduct by Edwards Lifesciences could breach EU antitrust rules that prohibit abuses of a dominant market position (Article 102 of the Treaty on the Functioning of the European Union).
The reason for the investigations lied in the fact, that the Commission had concerns that the inspected company may have violated EU antitrust rules that prohibit abuses of a dominant market position (Article 102 of the Treaty on the Functioning of the European Union). The Commission officials were accompanied by their counterparts from the national competition authority of the Member State where the inspection was carried out.
Unannounced inspections are a preliminary investigative step into suspected anticompetitive practices. The fact that the Commission carries out such inspections does not mean that the company in question is guilty of anti-competitive behaviour nor does it prejudge the outcome of the investigation itself. The Commission respects the rights of defence, in particular the right of companies to be heard in antitrust proceedings.
There is no legal deadline to complete inquiries into anticompetitive conduct. Their duration depends on a number of factors, including the complexity of each case, the extent to which the companies concerned co-operate with the Commission and the parties’ exercise of their rights of defence.
The Commission has in particular been investigating whether Edwards Lifesciences, through its UPIP, may have limited physicians’ freedom to participate in clinical trials and other scientific and educational activities sponsored or supported by a competing manufacturer of Transcatheter Aortic Valve Implantation (TAVI) devices. By limiting that competitor’s access to important services which assisted their research on medical devices, such as input derived from said physicians, Edwards Lifesciences could have made it more difficult for such a competitor to establish its products in the European Economic Area.
After thorough analysis and careful assessment of all evidence gathered and in light of Edwards Lifesciences’ withdrawal of the UPIP, the Commission has concluded that the investigated concerns have been addressed and further action is no longer considered a priority at EU level.
The closure of the investigation is not a finding that the conduct in question complied with EU competition rules.
Background: Edwards Lifesciences is a medical technology company headquartered in the US, which focuses on medical devices for cardiovascular applications.
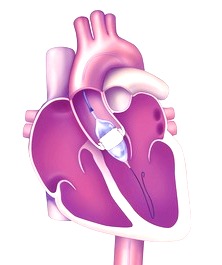
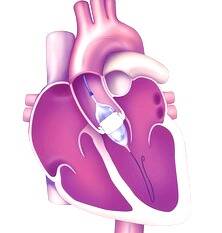
Transcatheter Aortic Valve Implantation procedures make it possible to replace aortic valves with an artificial valve implanted via catheter, thus avoiding putting the patient through a complex open-heart surgery. Edwards Life science’s TAVI devices are used to treat aortic stenosis, one of the most common and serious cardiovascular diseases.
Cardiovascular diseases are the leading cause of premature death in the EU. On 16th December 2025, the Commission adopted its ‘Safe Hearts Plan’, which includes measures to improve prevention, detection and treatment of cardiovascular diseases.
Safe Hearts Plan –
Cardiovascular diseases are the leading cause of premature death in the EU and they are preventable. They kill 1.7 million Europeans every year. Without urgent action, cardiovascular diseases are projected to rise by 90% by 2050. Furthermore, cardiovascular diseases cost the European economy €282 billion annually.
The Safe Hearts Plan is the first ever comprehensive EU approach to tackling this immense public health challenge. It presents targeted measures to improve prevention, detection and treatment of cardiovascular diseases.
The Plan improves heart health by helping individuals with personalised disease prediction tools and therapies, while addressing risk factors like tobacco, unhealthy diets, and alcohol. It seeks to bridge research gaps and integrate data, digital solutions and artificial intelligence to strengthen health systems. With levels of early cardiovascular deaths varying significantly across EU countries, the Plan emphasises reducing health inequalities and improving access to healthcare and therapies.
For example, the Commission will support Member States in developing national cardiovascular health plans, establish dashboards monitoring health inequalities, and launch an Incubator to speed up the use of AI. Beyond public health benefits, the Safe Hearts Plan also strives to bolster the EU economy and stimulate innovation in cardiovascular care, with clear goals set for 2035.
Team Maverick.
Providing Social Security and Economic Empowerment to Workers Is the State Government’s Priority – Smt. Lakshmi Rajwade
Raipur, February 2026 : A Shramik Jan Samvad and Conference programme was organised at the…