Imbalance of Nutrition affects Bone Growth and Height.
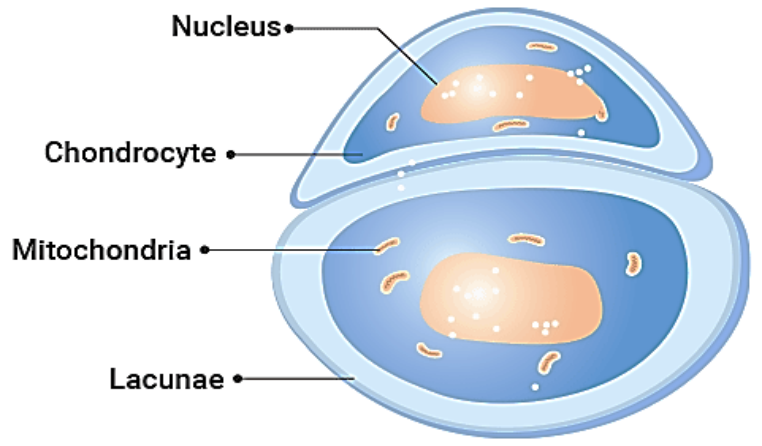
It is not any space science which have ascertained that Chondrocytes (cells responsible for producing and maintaining cartilage, a flexible tissue that cushions joints and provides structural support) play a crucial role in skeletal development.
Many bones, such as those in the arms and legs, are formed through endochondral ossification, in which chondrocytes assemble to create a cartilage scaffold that is later replaced by bone. The maturation of chondrocytes, required for their diverse functions, is regulated by epigenetic mechanism that modulate gene activity without altering DNA sequences.
Among these, DNA methylation is especially important, serving as a switch to suppress gene expression. Maintenance of proper DNA methylation requires the enzyme DNA methyltransferase 1 (Dnmt1).
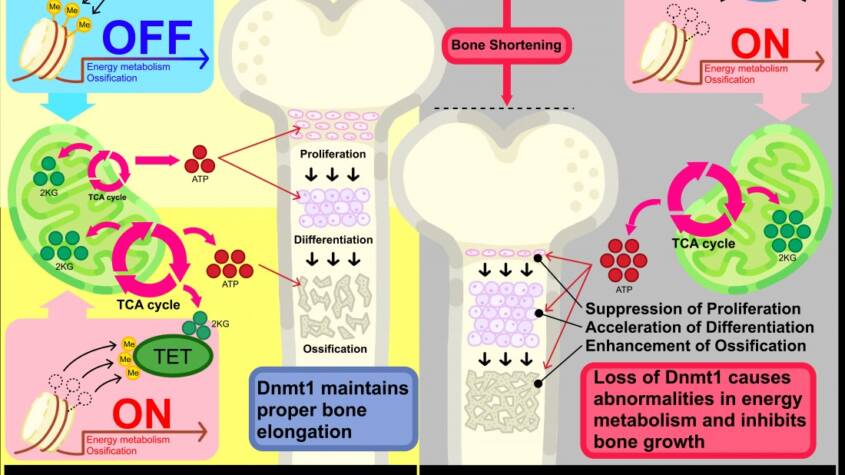
In this study, the function of Dnmt1 in bone growth was investigated. Using the musculoskeletal database “MSK-KP”, a strong association between genetic variation in DNMT1 and human height was identified. To examine its biological role, mice lacking Dnmt1 was generated specifically in mesenchymal progenitors of the limbs. These mutant mice displayed markedly shortened tibial length compared with controls. Detailed analysis revealed that Dnmt1 is expressed in the proliferative zone (PZ) of the growth plate cartilage, which is essential for longitudinal bone growth. Mutant mice showed reduced PZ, expanded hypertrophic zone (HZ), and accelerated calcification, indicating premature chondrocyte maturation.
To clarify the molecular basis, RNA sequencing and DNA methylation analysis of cartilage from mutant and control mice were performed. These experiments revealed that Dnmt1 directly regulates genes involved in cellular energy metabolism. Indeed, chondrocytes from mutant mice showed enhanced metabolic activity, and inhibition of metabolism suppressed the accelerated maturation phenotype. Furthermore, experiments using human chondrocytes isolated from surgical samples demonstrated similar results: reduction of Dnmt1 enhanced energy metabolism and increased markers of chondrocyte maturation, such as osteocalcin.
Taken together, these findings reveal that Dnmt1 regulates energy metabolism to ensure proper chondrocyte maturation and normal bone elongation. This discovery highlights the potential of nutritional and metabolic interventions in the prevention and treatment of impaired bone growth and osteoarthritis.
Dnmt1 regulates energy metabolism to ensure proper chondrocyte maturation and normal bone elongation. Loss of Dnmt1 disrupted normal DNA methylation in chondrocytes, leading to increased expression of genes involved in cellular energy metabolism. As a result, metabolic abnormalities occurred, promoting the differentiation and calcification of growth plate chondrocytes and ultimately inhibiting bone growth. These findings suggest that proper maintenance of DNA methylation mediated by Dnmt1 during chondrocyte differentiation regulates intracellular energy metabolism through gene expression control, thereby maintaining the balance of chondrocyte differentiation and calcification and ensuring normal bone growth.
However, when we discuss about the quintessential role of the Chondrocyte, similarly the exemplary function of the Chondroblasts needs to be highlighted.
Chondroblasts (perichondrial cells) are cells that play an important role in the formation of cartilage (chondrogenesis). They are located in the perichondrium, which is a layer of connective tissue that surrounds developing bone and also helps protect cartilage. Cartilage is the main type of connective tissue in the body and serves many functions.
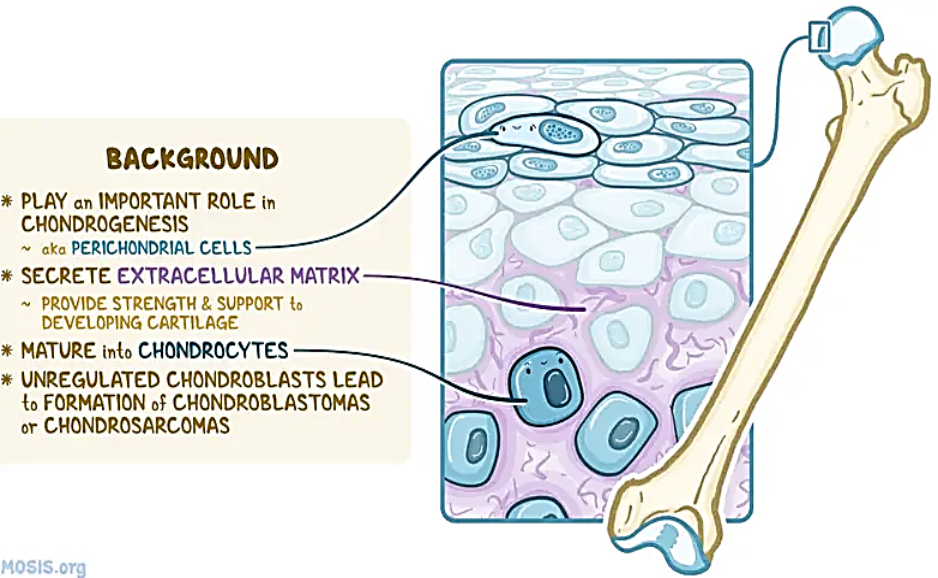
Chondroblasts contribute to the formation of the extracellular matrix and are the precursors of the chondrocytes, which collectively make up cartilage. Chondroblasts secrete the extracellular matrix which is composed of various substances, including collagen, proteoglycans, glycoproteins, hyaluronic acid, water, and macromolecules. These substances provide strength and structural support to the developing cartilage. In addition, chondroblasts mature into chondrocytes, which make up the cellular components of cartilage. These cells also contribute to the appositional growth of cartilage, which is characterised by the thickening of existing cartilage. They do this by secreting the extracellular matrix at the peripheral cartilage surfaces.
In existing cartilage, chondrocytes can be damaged or destroyed. When this happens, the remaining chondrocytes differentiate into chondroblasts in order to secrete more extracellular matrix and regenerate the lost cartilage tissue. However, this cartilage regeneration process is very slow, in part because of the lack of adequate blood supply.
In some cases, unregulated chondroblast growth and function can lead to the formation of chondroblastoma’s or chondrosarcomas. Chondroblastoma’s are benign tumors that form at endochondral ossification sites (places where growing cartilage is replaced by bone). They most commonly occur on the thigh bone (femur), shinbone (tibia), or humerus, located in the upper arm. On the other hand, chondrosarcomas are malignant tumors originating from the chondroblasts, and make up about 30% of bone cancer cases.
Team Maverick
PM Modi Presents Russian President Putin with Curated Gifts Reflecting India’s Culture and Heritage
New Delhi, Dec 2025: During the two-day visit of Russian President Vladimir Putin to India…